I hope you all know that running a car in poorly ventilated dyno cell can be a headache… literally. The cause of this is carbon monoxide poisoning.
Let’s quickly get through some basics. The process of fuel burning is a process of connecting of carbon atoms with the oxygen atoms in the air. If there is enough oxygen in the air to completely burn the fuel, one atom of carbon connects with two atoms of oxygen, creating CO₂ (carbon dioxide). CO₂ is not a problem for us. Our breathing process creates it too, and when there is too much of it, we have a natural reflex to breathe. Try inhaling CO₂ from a carbonated soft drink bottle after shaking it, and you’ll know what I mean. The situation is different when there is not enough oxygen to create CO₂ and one carbon atom connects with only one oxygen atom, creating CO (carbon monoxide). The problem with CO is that it binds to hemoglobin in our blood and blocks its ability to transfer oxygen. The other problem is that our body don’t detect it like in the case of CO₂. CO is colorless, odorless, tasteless. We can know that we inhaled too much when we already feel symptoms of poisoning like headache, dizziness, weakness, vomiting, chest pain, and confusion. If you breathe enough carbon monoxide quickly, then when you feel the symptoms you can be already on the way to the other side even if you immediately get out to get fresh air.
With internal combustion engines, the process of combustion gets a little twist. Even if there is enough oxygen around us to create CO₂, the engine takes in a portion of the air, and the ECU adds some fuel to precisely control the air-fuel ratio. If it creates a lean mixture, then it’s not that bad because we have enough air to create CO₂, however in motorsport we don’t want to waste any of these precious oxygen atoms that got into the engine, and we run the engine on a rich mixture that produces CO.
You heard a million times not to run the car in a closed garage. That’s obviously a good guideline, but for me, it’s missing some details about how fast it becomes dangerous. Since I couldn’t find any information about it on the internet and I recently started providing carbon monoxide sensors, I decided to find out myself and share this information with you.
For the experiments, I used a sports car with no catalytic converter.
Experiment 1 – carbon monoxide near exhaust
First, I want to know the concentration of CO directly behind the exhaust. I placed the sensor just behind the car and started the engine.

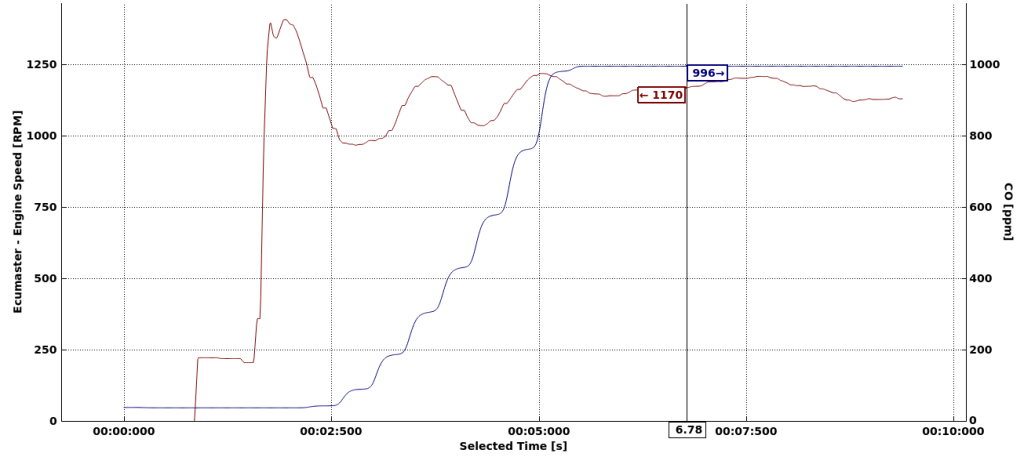
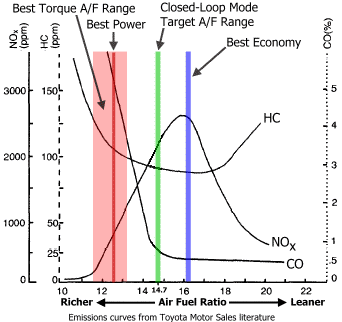
In 5.5 second the sensor reached its maximum range of 1000ppm. The car when starting runs on a rich mixture around 12 AFR. From the charts from Toyota we can read that will produce few ‰ of CO content, that is few thousand PPM. That’s consistent with my test results. Let’s say the exhaust fumes have 6000PPM then if you manage to breathe them in, then according to Wikipedia you will have: “Headache and dizziness in one to two minutes. Convulsions, respiratory arrest, and death in less than 20 minutes.” My conclusion from this is that you should definitely avoid standing behind the car on the dyno and being directly exposed to breathing exhaust fumes, no matter how good overall ventilation is in the dyno cell.
Experiment 2 – carbon monoxide buildup in closed garage
Now it’s time for the exciting part. How long does the car need to idle in a closed garage to get to dangerous levels?
The test was made in a closed garage of dimensions 6m by 7.3m, 2.7m high : total 118m³ (4000 cubic feet). The ventilation was disabled and all doors and gates were closed. Here is a picture of the location to give you some visual reference:

For this experiment, I placed the sensor on the roof of the car to represent exposure of a person in a similar location.

I also connected a lambda sensor to measure the oxygen concentration in the garage. I know it’s not the correct nor precise method to do it, but since I had the sensor on hand, why not log it.

The car was started and left idling.
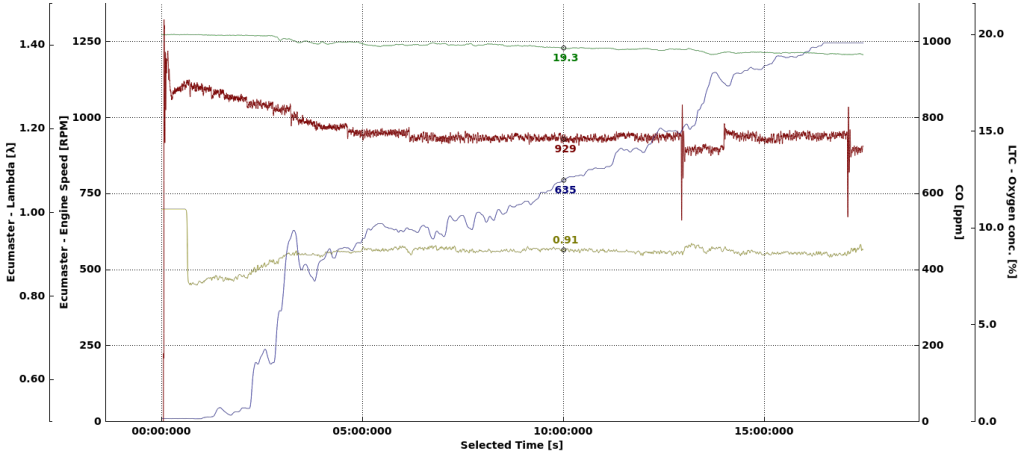
The CO level rises quite fast at the beginning. Probably due to richer mixture during engine warm up. After 3 minutes, the level is already over 400 PPM, which is enough to give you a serious headache if you spend 1–2 hours in these conditions. After 17 minutes we have full scale 1000PPM which is enough if you have suicidal intentions and another few hours of spare time to stay there.
Oxygen level in the garage does drop a bit, but not significantly. Again, the production of CO in this case is not a result of lack of oxygen in the garage, but the AFR of the mixture in the engine.
Final thoughts
It’s not that easy to get to the life-threatening CO levels while idling if you don’t intentionally close yourself with a car in the dyno cell for a long time. You may reach the dangerous level faster when driving on the dyno with some throttle, but still you will notice that you are in a room full of exhaust fumes and need to vent the cell.
On the other hand, exposing yourself to enough carbon monoxide to give you a headache after a dyno session is very easy if you don’t have a good ventilation system.
Wikipedia – Carbon monoxide poisoning
Exposure limits
* OSHA PEL
The current Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) for carbon monoxide is 50 parts per million (ppm) as an 8-hour time-weighted average (TWA) concentration [29 CFR Table Z-1].
* NIOSH REL
The National Institute for Occupational Safety and Health (NIOSH) has established a recommended exposure limit (REL) for carbon monoxide of 35 ppm as an 8-hour TWA and 200 ppm as a ceiling [NIOSH 1992].
* ACGIH TLV
The American Conference of Governmental Industrial Hygienists (ACGIH) has assigned carbon monoxide a threshold limit value (TLV) of 25 ppm as a TWA for a normal 8-hour workday and a 40-hour workweek [ACGIH 1994, p. 15].
